Projet ANR-21-CE21-0004
Coordinator: Université Bourgogne Franche-Comté
Period: 01/2022 - 12/2025
Partner : Université Bourgogne Franche-Comté (UMR 1231), INRAe (UMR SayFood 0872), LNE, Institut Pasteur de Lille.
Context
The French AGEC law (law n°2020-105) relating to the fight against waste and the circular economy imposes the recycling of all plastics from 1er January 2025, which is highly impactful for the food contact materials (FCM) sector. The target is ambitious, but in line with the European policy set by Directive (EC) no. 2019/904: reuse of packaging (10% of packaging by 2027) and recycled materials (30% of recycled materials by 2030).
The introduction of recycled material calls into question the principles of purity of the plastics used (monomers and additives) and the migration concepts used to assess them (positive list, specific migration limit) according to Regulation (EC) No. 10/2011.
The widespread use of recycled materials introduces new problems that were not envisaged in the founding texts: extreme variability of recycled batches, the use of materials that have undergone several recycling cycles, mixed sourcing (recycled food contact and non-food contact applications (internal or external to the EU), the combination of non-decontaminated and decontaminated materials (for example, used behind a functional barrier made of recycled but non-decontaminated material) and the use of several types of material in mixtures or complexes. The situation is becoming even more complex with the increasing use of combinations of cellulose materials and recycled plastics. The lack of standards between materials, between member states, virgin and recycled materials, and the fragmentation of supply chains (collectors, recyclers, processors, importers) make it impossible to guarantee homogeneous food safety The mineral oil crisis and the widespread contamination of foodstuffs by a Potentially carcinogenic aromatic substances illustrate the absolute need to modify current risk management procedures with effective safety assessment methods applicable to complex mixtures containing unknown contaminants.
Objectives
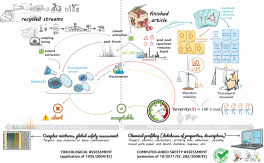
The central idea of the PackSafe project is to simultaneously manage all substances present in an MCDA and likely to migrate to food for an integrated safety assessment to ensure that the MCDA does not release any hazardous chemicals.
To this end, the project aims to develop a generic methodology for assessing the safety of MCDAs containing IAS (intentionally added substances) and NIAS (non-intentionally added substances), whatever their origin and according to several toxicological parameters. The central objective is to optimize material extraction conditions (plastics, paper/cardboard, composites) to standardize and make reliable in vitro biotests. Biotest interpretation will be coupled with non-targeted chemical analysis and data processing and migration modeling for an integrated safety assessment of complex extracts. This research will support a need for future evolution of EU regulation on MCDA.
Methodology
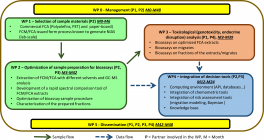
The project is divided into six complementary tasks (T0..T5) as summarized in the figure below.
Biotests are currently used to assess the safety of MCDAs (cardboards, polymers, glass, adhesives,) in terms of cytotoxic/genotoxic activities and potential endocrine disruption. They provide additional information compared with NIAS analysis, insofar as they are able to capture the cocktail effects obtained by interactions between chemicals in extracts. However, they need to be better standardized for specific use with MCDAs to avoid "false answers" due to the complex composition of the extract.
Sample production (WP1) and sample preparation (WP2), which requires a concentration step, are key stages in the successful use of biotests. Indeed, insufficient solubility and the problem of quenching at high oligomer levels can lead to erroneous results. A technique for extracting materials using biocompatible solvents will be developed. To enhance the sensitivity and robustness of bioassays, a new sample preparation methodology for extract cleaning and fractionation will be generated. In order to ensure that extracts are representative, chemical fingerprinting of extracts will be carried out using various analytical techniques (1H NMR, GC-MS, LC-MS, etc.).
Task WP3 aims to identify the most appropriate biotest(s) according to the toxicological endpoint under study (cyto/genotoxicity, hormonal activities for endocrine disruption). The detection threshold of the biotest must be determined according to its specificity and sensitivity. Production of relevant toxicological data in parallel with chemical spectra analysis will be carried out on representative extracts and fractions to feed the database.
Compound detection and associated risk assessment will be carried out in a modular, programmable open source environment (WP4). The environment will be created by bringing together numerous proven tools and already consolidated databases. Developments will focus on the design of pipelines and application interfaces between currently incompatible modules, and on the construction of a global Bayesian approach to severity scaling.
Expected results
As expected results, a decision toolbox will be distributed as an open-source project with databases and digital tools. These efficient and cost-effective tools will help the packaging and food industries to guarantee the safety of their materials, comply with recent MCDA regulations and provide a rapid response to the expected market shift towards recycled materials, biodegradable or reused packaging.
The holistic and pragmatic approach using strategy (migration/hazard identification/chemical identification) for MCDA safety will also increase innovation as causalities in NIAS generation can be described and then avoided. A new strategy leading to a decision-tree approach to NIAS risk assessment will be scientifically developed, underpinning guidance documents for national and European agencies. It will help public bodies make decisions in complex situations and cope with uncertainty.
Impacts
Used upstream of packaging design, these tools will encourage innovation (Safe-by-Design) and ensure a gain in competitiveness for the packaging sector, food companies and recyclers. Faster safety assessment can bring innovations to market more quickly. The methodology developed could be generalized to outside fields where the hazard of unknown substances in complex mixtures needs to be assessed, such as the pharmaceutical industry (e.g. plant extracts), medical device suppliers, cosmetics. The expected outcome of the project is the extension of fundamental scientific knowledge on NIAS and the development of an integrated chemical and toxicological approach based on expertise in packaging technology, analytical chemistry with innovative methods of data processing and hazard assessment.
Communications
7th edition of the ILSI conference
Poster
Severin, I.; Nguyen, P.-M.; Platel, A.; Lyathaud, C.; Julien, J. M.; Kermorvant, J.; Moche, H.; Vitrac, O.; Domenek, S.; Chagnon, M.-.C. PACKSAFE: Integrated chemical safety assessment of food contact articles (ANR-21-CE21-0004 project: 2022-2025). 7th International Packaging Materials Symposium (ILSI), May 3-6. Digital event, 2022.