In the cosmetics sector, Regulation (EC) No. 1223/2009 defines the regulatory requirements for products placed on the market. Thus in accordance with Article 3 of the regulation, cosmetic products must not endanger human health under normal or reasonably foreseeable conditions of use. According to Article 10 of the regulation, proof of compliance with this requirement lies with the person responsible for the finished product, who must carry out a safety assessment on the basis of appropriate information. Article 17 of the regulation stipulates that the unintentional presence of prohibited substances must be assessed, in particular those resulting from packaging migration, and Annex 4 of Regulation (EC) No. 1223/2009 states that the relevant characteristics of the packaging material must be taken into account in the safety assessment, in particular its purity and stability. Although the European Commission has published guidelines for the implementation of these requirements (Implementing Decision of 25/11/2013), the evidence to be provided by those responsible for placing products on the market is not perfectly established. So what approach should be adopted to verify the health safety of cosmetics packaging?
Transposing regulatory requirements for food contact materials
In the agri-food sector, partially harmonized regulations exist for materials and packaging intended for contact with foodstuffs (Regulation (EC) no. 1935/2004). This regulation requires that all materials and articles intended to come into contact, directly or indirectly, with foodstuffs be sufficiently inert not to transfer to these foodstuffs constituents in a quantity likely to present a danger to human health, to bring about an unacceptable change in the composition of the foodstuffs or to alter their organoleptic characteristics. The food industry approach can be transposed to the cosmetics industry. With the exception of skin sensitivity, it is reasonable to assume that exposure to chemical substances through skin contact is no more dangerous than exposure through ingestion. Thus, subject to a level of exposure that is at most equivalent, depending on the exchange surfaces between packaging materials and cosmetic products, the migration limits adopted for food packaging (SMLs) can be just as safe in the case of cosmetics packaging. On this basis, assessing the safety of cosmetic products involves taking into account the specificity of these products, in terms of their physical and chemical properties and their conditions of use, in order to best adapt protocols for analyzing substance migration.
.Substance transfer from packaging materials to cosmetics
Migration is the generic term generally used to designate the transfer of a chemical substance or several chemical substances from a packaging material to a contained product (a cosmetic product, for example).
In the case of plastics and, more generally, polymers, those involved in the food industry use two terms to designate migration (see EU regulation no. 10/2011):
- overall migration is defined as the maximum amount of non-volatile substances that migrate into food, also known as "migrat". This is a material property, and the accepted threshold is independent of the migrat's toxicity.
- specific migration is defined as the amount of a particular substance that migrates into food, also referred to as a migrat, and whose accepted threshold is in principle determined from the known toxicity of the substance.
The transfer of chemical substances from polymers to the products contained mainly involves sorption/desorption, diffusion and convection. Substance movement, a consequence of molecular agitation (Brownian motion), will generate flows of molecules producing substance transfer in both directions, either from the material to the contained product, or from the contained product to the material. At a given temperature, the diffusion of a substance in a matrix follows Fick's laws and is characterized by its diffusion coefficient D (in m²/s). Transfer kinetics involve an initial phase in which the concentration of the migrant in the matrix increases at a rate dependent on the diffusion coefficient. During a second phase, thermodynamic equilibrium is reached when the flux of molecules migrating from the material to the matrix is equal to the flux of molecules migrating from the matrix to the material. The distribution of a substance between a material and a contained product is represented by its partition coefficient K (a unitless quantity which is the ratio of the volumetric concentrations of the substance at equilibrium between the material and the contained product).
The limits of the substance migration analysis approach
To assess the health safety of materials and packaging intended for contact with cosmetic products, conventional means and strategies using analytical methods to measure migration have limitations that don't always allow the demonstration to be taken to its logical conclusion. Few methods for measuring specific migration have been standardized, and the cost to laboratories of developing new methods is high. Most of the time, analyses are carried out on the basis of the rules laid down by the regulations for plastics intended for food contact, using standardized conditions that do not correspond exactly to actual conditions of use.
To complement laboratory analysis approaches, since 2002 the European Union has accepted, in the agri-food sector, that the assessment of a substance's potential migration can be calculated by applying generally recognized diffusion models, based on scientific data, and established in such a way as to overestimate actual migration.
Modeling is accepted if it meets two conditions:
- the substance must first be identified;
- the final concentration in the feed or the quantity transferred into the feed must be overestimated by the calculation tool compared with the real situation or compared with the result of a migration analysis.
On this basis, INRA has launched research involving LNE and other partners, which has led to the development of new approaches and tools aimed at better demonstrating the sanitary safety of packaging. These approaches could also be used in the cosmetics sector. They are based on substance transfer modeling applications such as SFPP3 ("Safe Food Packaging Portal" Version 3) accessible on the INRA website [1].
The substance transfer modeling approach
Substance transfer modeling is based on simplifying assumptions allowing
predict the migration of a substance from a material to the contained product, such as a cosmetic product. These assumptions and recommendations are summarized in the European Commission's guide for demonstrating the conformity of food contact materials. The ultimate aim is to predict the amount of substance released into the contained product after a given contact time, an approach that can be used by extension to cosmetics packaging.
Based on the phenomena involved in the transfer of chemical substances, the modeling tool developed by INRA can predict the contamination of a matrix. In the case of cosmetic products, the input data for the calculation involves the characteristics of the packaging, the transfer properties of the substance and the conditions of use, i.e.:
- the contact surface of the packaging with the cosmetic product;
- the concentration of the substance in the material;
- the volume of material and its density;
- the volume and density of the cosmetic product;
- the diffusion coefficient of the substance in the material and in the cosmetic product at a given temperature;
- the partition coefficient of the substance between the material and the cosmetic product;
- the contact times and temperatures between the material and the cosmetic product.
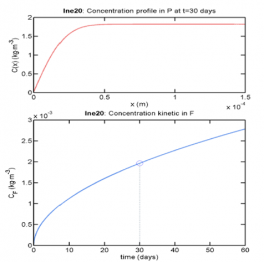
The results of the modeling calculation are presented in the form of a curve (see Figure 1) showing the migration kinetics (concentration of the substance evaluated in the cosmetic product as a function of the contact time with the material) and in the form of a profile of substance concentration as a function of material thickness after the chosen contact time with the cosmetic product. With these results, it is possible to predict the contamination of cosmetic products by substances, particularly at the expiration date of the packaging. The migration values obtained can then be compared with the regulatory thresholds accepted in the food industry (SML in mg/kg of food).
The transfer modeling approach is already used by LNE to assess the safety of food packaging, and can be extended to cosmetics packaging.
This approach simplifies the estimation of substance migration kinetics under conditions as close as possible to actual product use. Results are thus available in a matter of days for cosmetic products stored in their packaging for several months or even years. What's more, assessment of the migration of materials into cosmetic products is possible even for substances not available from laboratory product suppliers, which is often the case for neoformed substances, or where analytical protocols have not yet been developed.
Useful links
Update date: August 2016
Would you like us to help you address the health and safety of your materials?
food contact specialist
To help you:
- Know the regulatory requirements for materials and articles intended to come into contact with foodstuffs;
- Provide proof of compliance with these requirements;
- Integrate food safety concepts into innovation and research / development.
LNE offers solutions and support in all phases from packaging design to production, and adapts methodologies and solutions to your needs.
Discover our services: technical assistance/consulting/engineering, monitoring, analyses, training, certification, r&d